Partner with Indero’s Project Management Team
Indero has over 25 years of experience in meeting sponsor expectations with transparent, collaborative clinical trial project management. Tell us about your study today.
Proactive clinical trial project management services for dermatology and rheumatology studies. We are committed to excellence: Delivering timely, cost-effective, and fully compliant solutions.
At Indero, our project managers (PMs) are the driving force behind your project’s success, acting as the CEO of your project. From the earliest stages of development to the final phases, they ensure every aspect of your project is meticulously managed. They oversee every detail by proactively monitoring risks and driving enrollment strategies to ensure on-time, high quality, high standards, and cost effective study delivery.
With experience in dermatology, rheumatology, and other specific indications, we assign a PM and team with the appropriate expertise to manage your project effectively. From the onset, they assess risks, implement mitigation plans, and continuously monitor and adjust as needed.
Our project directors (PDs) provide oversight and white glove service throughout the project, ensuring all cross-functional teams are aligned and performing to exceed client expectations. Additionally, our hands-on and accessible executives are not afraid to roll up their sleeves and get involved, leading with frontline focus in mind.

Our partnership-driven methodology ensures that our project management team operates as an extension of your team, fostering seamless alignment between clients, sites, and cross-functional teams. With agility and adaptability, we tailor strategies to meet evolving study needs, optimize workflows, and enhance efficiency. Through proactive engagement and open communication, we transform collaboration into a competitive advantage, delivering faster, smarter, and more successful trials.
Indero’s project management team is an ideal mix of project directors, project managers, and other key operation specialists, with experience and expertise in dermatology and rheumatology indications from early to late stage clinical trials. Our teams receive extensive training from our collection of specialized medical and scientific affairs team members.

The Dermatology Center of Indiana and the Indiana Clinical Trials Center

“Our project manager was fantastic! The entire team was very knowledgeable, collaborative, and responsive.”
With a dedicated PM and PD serving as your primary points of contact, we ensure seamless communication and swift issue resolution. Our escalation framework is designed to address challenges proactively, enabling rapid decision-making and minimizing delays. The PM drives day-to-day operations with precision, while the PD provides strategic oversight and leadership, ensuring your study stays on track, on budget, and aligned with your corporate goals. Together, they deliver a partnership built on accountability, transparency, and results.

Indero Project Director
Unique to Indero, our PDs oversee client projects from proposal to closure, ensuring a seamless end-to-end approach. Significant issues are escalated to the study sponsor within 24 hours.

Indero Project Manager
First escalation point for all project-related topics
We have operational hubs across Europe and North America with capabilities spanning Europe, North America, Asia Pacific, and Latin America. Our team speaks the local languages to enhance communications with sites and foster strong relationships.
At Indero, we are on top of the wave when it comes to leveraging cutting-edge technology to drive efficiency and transparency in our project management processes.
Technology-Driven Efficiency
Embracing the latest technological advancements, we eliminate manual processes and streamline our operations. We use the finest CTMS and EDC systems for accurate data collection, remote monitoring technologies to oversee trials efficiently, and wearable devices for continuous subject monitoring.
PowerBI Dashboards
Our real-time data dashboards provide comprehensive and up-to-date visualizations of our project metrics.
Data Lake Integration
All our data is fed from a centralized data lake, ensuring that we have a single source of truth.
Indero has over 25 years of experience in meeting sponsor expectations with transparent, collaborative clinical trial project management. Tell us about your study today.

Rated Indero “Satisfactory” for the experience*
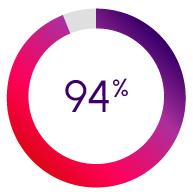
Attributed an overall rating of 4 out of 5 or more

Rated Indero “Satisfactory” for responsiveness to study request*
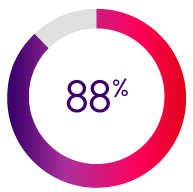
Rated Indero “Satisfactory” for overall governance*

Rated Indero “Satisfactory” for project team structure*

Rated Indero “Satisfactory” for handling important ad-hoc problems*
Scientific discipline. Operational efficiency. Clinical development expertise. Our project management services provide the rigorous foundation, quality data, and expertise dermatology and rheumatology studies need to ensure effective execution and on-time delivery.
“We have been very pleased with the team collaboration and Indero’s proactiveness as well as thorough and complete assessment of issues and risks, with detailed options and recommendations.”
“They seem to care about the projects and are open to hearing suggestions and feedback.”
Wherever
You’re Going,
We’re all in.
Need help with your next study? We want to learn more. Fill out the form on the right and one of our experts will be in touch.
3530 Saint-Laurent Boulevard
Suite 300
Montreal, QC, H2X 2V1, Canada
© 2025 Indero. All Rights Reserved. Privacy Policy. Designed and developed by Altitude Marketing.

Our long-standing relationships with key opinion leaders and highly performing sites result in optimized start-up processes that deliver strong results in the least amount of time. As part of our site evaluation, our team utilizes past performance metrics, recruitment projections based on the competitive landscape, and detailed feasibility surveys to determine a site’s capabilities and interest in a study.

Our dedicated patient recruitment and advertising team integrates with the study team to tailor recruitment strategies, customize central ad campaigns, develop advertising creative, and continuously track and adapt recruitment goals. Our patient-centric approach is enhanced by Clinago, our technology enabled recruitment service.

We orchestrate all study activities and deliverables, including the site selection process, budget and contract negotiations, patient engagement, and recruitment enrollment projections, all while respecting your study timelines.

We orchestrate all study activities and deliverables, including the site selection process, budget and contract negotiations, patient engagement, and recruitment enrollment projections, all while respecting your study timelines.