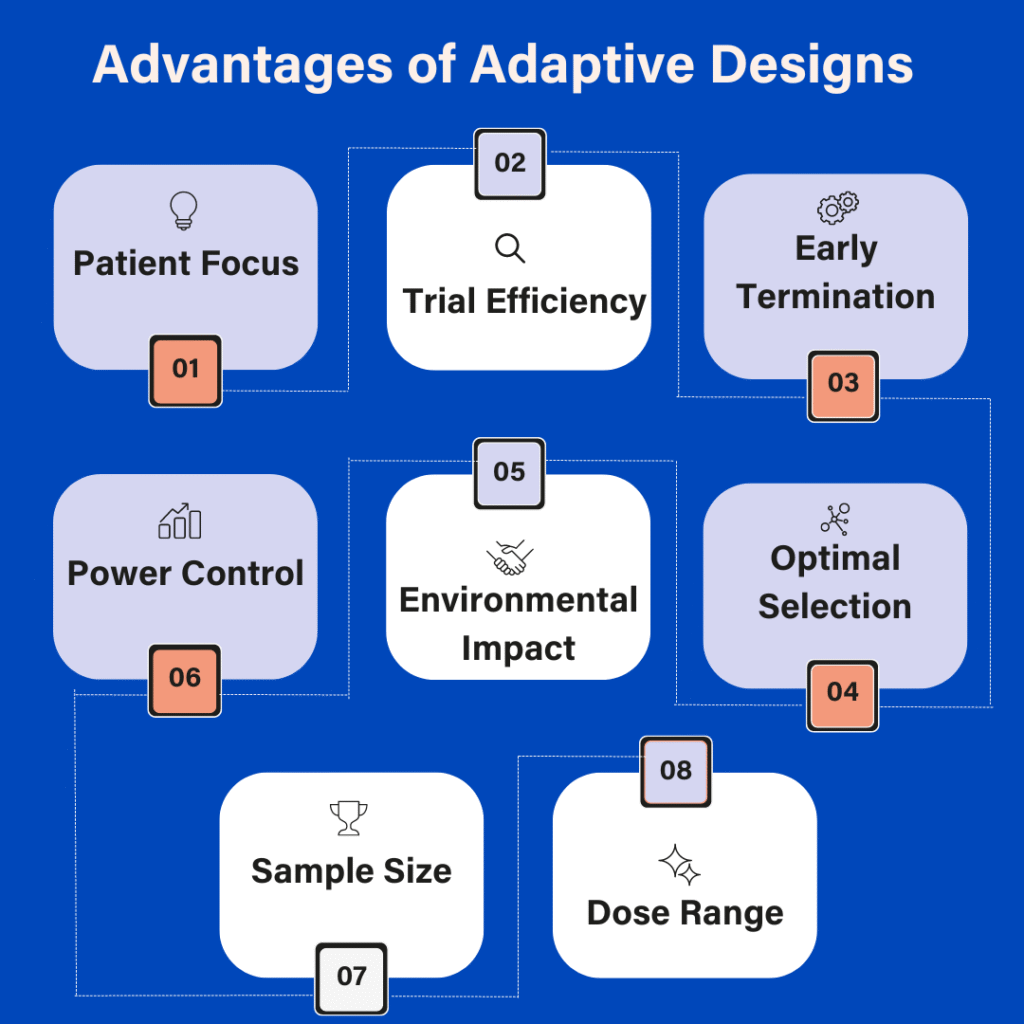
 Explore our latest infographic to get a comprehensive overview of the advantages of adaptive design.
Explore our latest infographic to get a comprehensive overview of the advantages of adaptive design.
- Patient Focus
Limiting patient exposure to drugs or doses that are not sufficiently effective. - Trial Efficiency
Allowing trial completion with fewer patients, enriching the trial population in a subgroup of patients for whom the medication is effective. - Early Termination
Stopping the trial early if the medication is not effective. - Optimal Selection
Selecting a dose, a formulation or a treatment regimen for which the efficacy is good enough to be approved by health authorities. - Environmental Impact
Reducing the environmental impact of clinical research. - Power Control
In contrast, increasing the sample size to maintain or achieve the trial power could also prevent a study from failing for lack of power, and thus, have a better control of the Type 2 error. - Sample Size
Adjusting, especially reducing, the required sample size needed may be crucial for a study conducted in a rare disease, or a disease that is more difficult to recruit due to comorbidities. - Dose Range
Drug developers may study a wider range of doses.
Click this link to download the PDF version.
Read the full article: Potvin D, D’Angelo P, Bennett S, Jankicevic J, Bissonnette R. Adaptive designs in dermatology clinical trials: Current status and future perspectives. J Eur Acad Dermatol Venereol. 2024 Apr 15. doi: 10.1111/jdv.20030. Epub ahead of print. PMID: 38619384.



