Click here for a PDF version of this case study
Outcomes
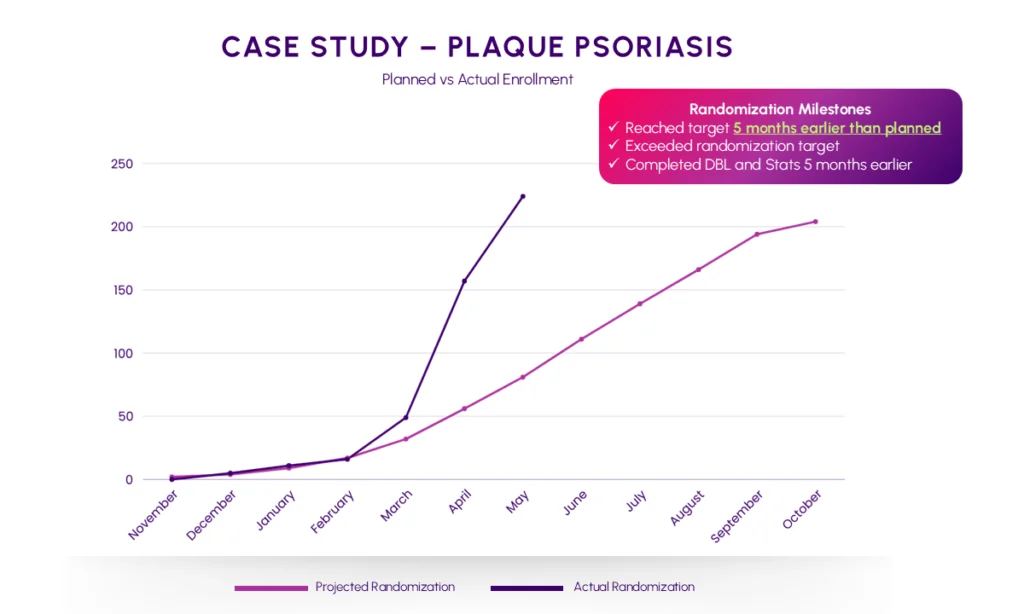
Indero successfully managed this plaque psoriasis study and this resulted in:
Enrollment completion 5 months earlier than planned
Study Characteristics
- Study Phase: II
- Patient Population: Plaque Psoriasis
- IP Route of Administration: Systemic
- Total Enrollment: 224 patients, 59 sites (North America & Europe)
Complex Study Procedures
- TBNK / hsCRP: Non-frequent procedure necessitated collaboration with central lab to customize the kits and lab manual
- Genetic testing
- Novel take-home photography equipment
Study Challenges and Solutions
Challenges #1
IP Availability: Sponsor decided to initiate the study even if there was no sufficient IP available to cover all regions
Solutions
- Indero adapted the country and site activation strategy accordingly
- Activated less European sites than expected
- Careful monitoring of the number of patients enrolled per site and regular communication with the sites
Challenge #2
IP Stability: IP stability data confirmed the drug’s expiry date could be extended, which required sites to relabel the IP up to 3 times, due to study duration
Solution
- Indero shared specific relabeling procedures with sites and supported them during the process, ensuring impeccable documentation
Recruitment Curve
Strategies for Success
- Strong recruitment strategies were established due to expected recruitment challenges
- LTE and OLE parts were added to protocol
- Opened recruitment in Europe, in addition of North America
Need help with your next dermatology or rheumatology study? Tell us a little about your needs.




