Click here for a PDF version of this case study
Outcomes
Indero successfully rescued this urticaria study and this resulted in:
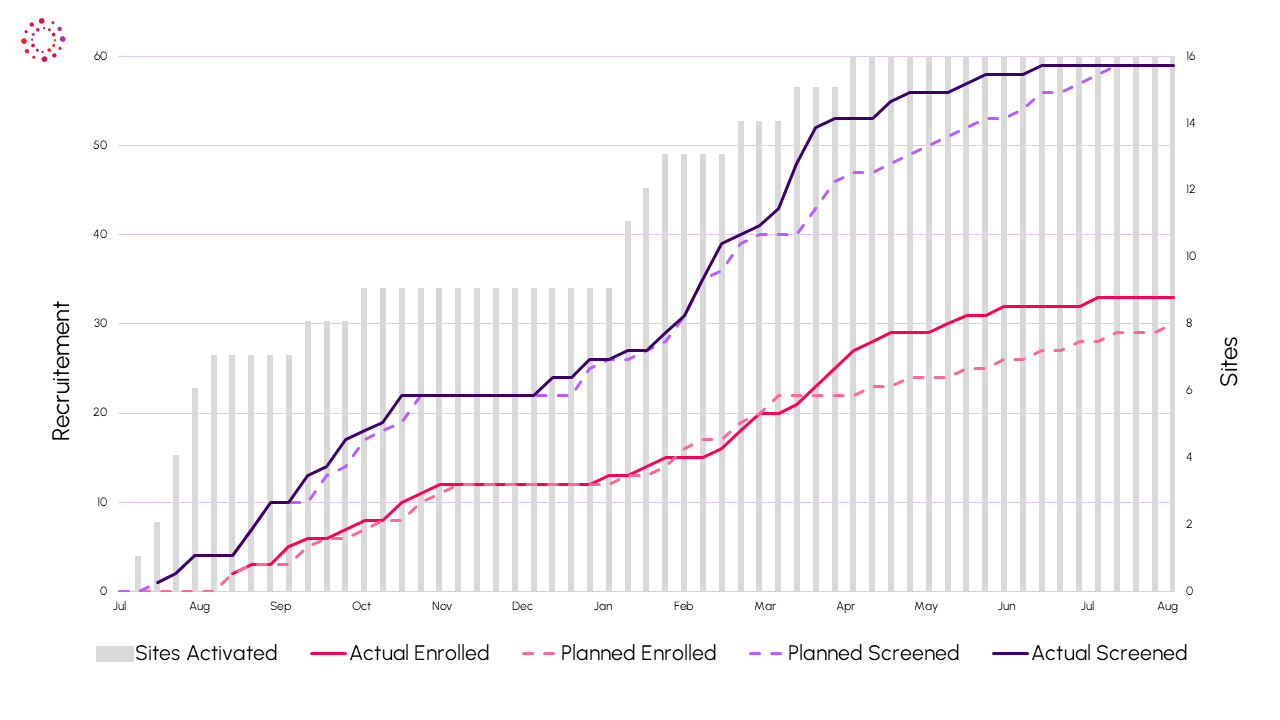
Recruitment on Time
The study was completed successfully, demonstrating Indero’s ability to manage early phase international clinical trials.
Client Feedback
“We have had strong project director support which contributed to the successful collaboration between the client and Indero. Our concerns and issues were heard and addressed in a timely manner.”
Study Details
- Phase: Ib
- Indication: CIndU (Cold urticaria or Symptomatic dermographism)
- Sites Location: Germany (4), Netherlands (1), Spain (2), Canada & USA (9)
- Blinding: Open Label
Active Collaboration
- Strong Relationship with Charité: The collaboration with Charité and Dr. Metz was instrumental in achieving the study’s objectives.
- Successful IM in Berlin: High engagement from the sites and excellent representation from across Europe.
Challenges & Solutions
- Seasonality: Despite key recruitment period over warmer months with reduced prominence of this indication, recruitment completed on time with 48% of subjects from EU.
- Coordination: Effective coordination and problem-solving were crucial in overcoming these challenges.
Need help with your next dermatology or rheumatology study? Tell us a little about your needs.




