 Adaptive design in clinical trials is a dynamic and innovative approach, but it requires certain key elements to ensure its successful implementation.
Adaptive design in clinical trials is a dynamic and innovative approach, but it requires certain key elements to ensure its successful implementation.
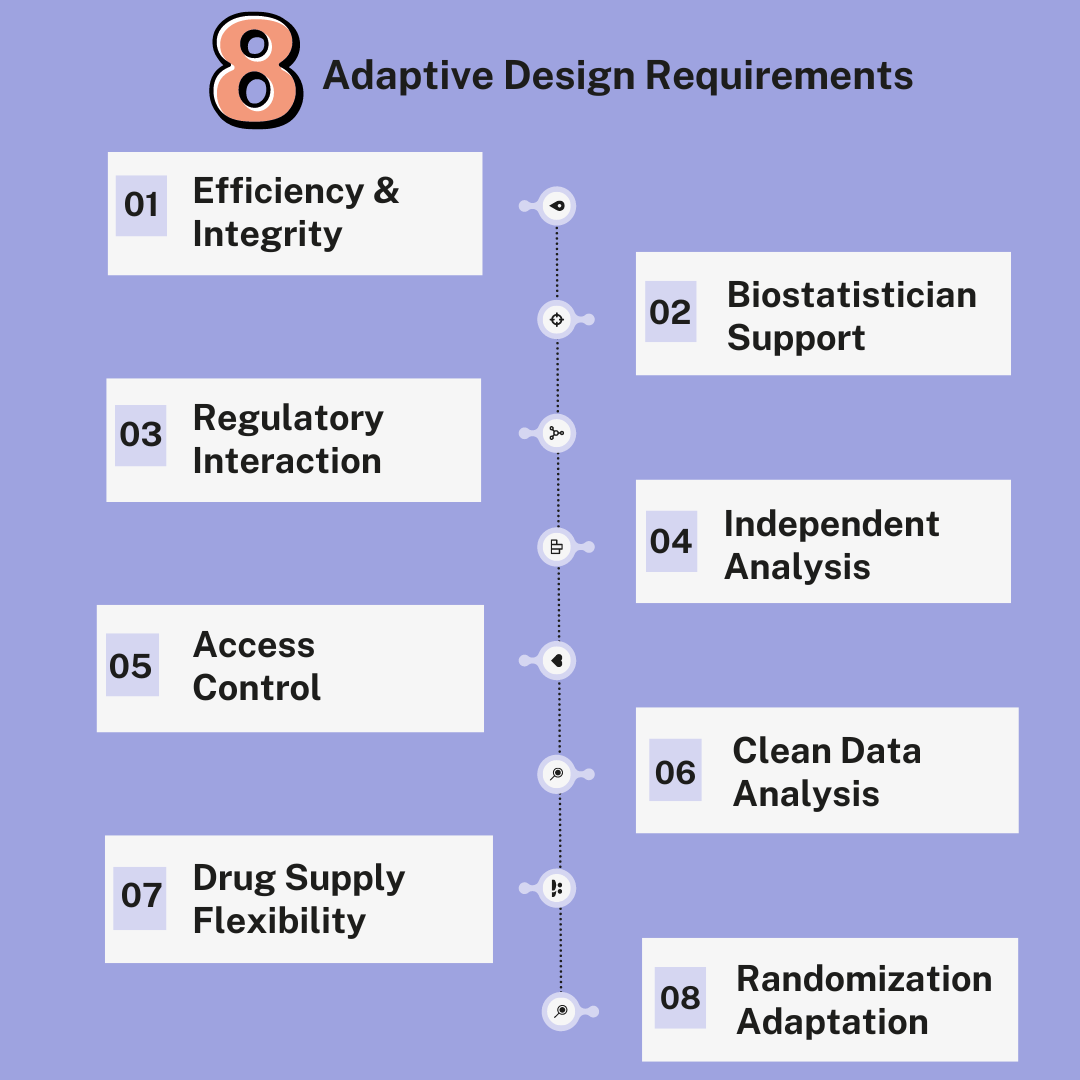
- Efficiency and Integrity
The purpose of an adaptive trial design is to increase efficiency without compromising the integrity and validity of the results. For this to be accomplished, extensive and adequately maintained clinical trial planning is required. - Biostatistician Support
More statistical support from a biostatistician that is well versed in adaptive trial design methodologies is needed before the trial is initiated. - Regulatory Interaction
Adaptive trial designs also typically require more interaction with regulatory bodies prior to initiating the trial to establish agreement on the proposed strategies. - Independent Analysis
They also generally require the use of a separate and independent group of biostatisticians and programmers who will only be involved in the interim analyses, particularly for unblinded adaptations. - Access Control
Access to the repository of documents, programs and data must be available to an unblinded group only and the minimally required information should be shared with the blinded team members following adaptive modifications to avoid bias. - Clean Data Analysis
Monitoring of patient data generated up to the interim analysis timepoint must be completed and the data hard locked (or soft locked depending on the scope of the interim analysis) before performing the interim analysis. This requires more time and effort from the clinical operations team. - Drug Supply Flexibility
Adaptive trial designs also often require a good and flexible drug supply, especially if one or more cohorts need to be expanded or terminated. - Randomization Adaptation
By employing a response-adaptive randomization, an adaptive trial design may also modify the randomization scheme, which allows changes to the randomization probabilities. This means shifting the randomization probabilities in favour of arms that showed promise during the course of the trial or stopping poorly performing arms altogether (i.e. effectively reducing their randomization probability to zero). This implies more complex randomization strategies and programming, which may incur a cost and more potential for error.
Click this link to download the PDF version.
Read the full article: Potvin D, D’Angelo P, Bennett S, Jankicevic J, Bissonnette R. Adaptive designs in dermatology clinical trials: Current status and future perspectives. J Eur Acad Dermatol Venereol. 2024 Apr 15. doi: 10.1111/jdv.20030. Epub ahead of print. PMID: 38619384.